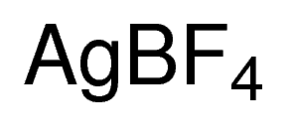Silver tetrafluoroborate is an inorganic compound with the chemical formula AgBF4. It is a white solid, although commercial samples often are gray, that dissolves in polar organic solvents as well as water.
Preparation
Silver tetrafluoroborate can be prepared by several methods. A simple route entails dissolving silver carbonate in aqueous tetrafluoroboric acid. It can also be produced by treating silver(I) fluoride with boron trifluoride in nitromethane solution. The reaction between boron trifluoride and a benzene suspension of silver oxide is yet another route, one that exploits the solubility of the compound in benzene. This method however affords silver fulminate, a sensitive explosive.
Laboratory uses
In the inorganic and organometallic chemistry laboratory, silver tetrafluoroborate, sometimes referred to "silver BF-4", is a used as a reagent to remove halide ligands and to oxidize electron-rich complexes. In dichloromethane, silver tetrafluoroborate is a moderately strong oxidant. Similar to silver hexafluorophosphate, it is commonly used to replace halide anions or ligands with the weakly coordinating tetrafluoroborate anions. The abstraction of the halide is driven by the precipitation of the corresponding silver halide.
Structure
According to X-ray crystallography, the solid compound consists of Ag centers bound to four fluoride sites of the BF4−.
References